News Get the latest updates on everything here at KORdotSIN
ListRussia's COVID-19 VACCINE???
- Author KORDOTSIN
- Date20-08-13 10:22
- Views 4,862
- Reply 2
Link
Main text

With talks of vaccines only coming earliest by the end of the year, countries are ramping up their vaccine research efforts.
Russia, however, shocked the world by becoming the first country to approve a COVID-19 vaccine.
But is it all that it seems?
Read on from this Straits Times excerpt to find out!
An announcement by Russia on Tuesday (Aug 11) that it will approve a COVID-19 vaccine after less than two months of human testing prompted alarm among global health experts, who said that with no full trial data, the vaccine is hard to trust.
Intent on being first in the global race to develop a vaccine against the pandemic disease, Russia has yet to conduct large-scale trials of the shot that would produce data to show whether it works - something immunologists and infectious disease experts say could be a "reckless" step.
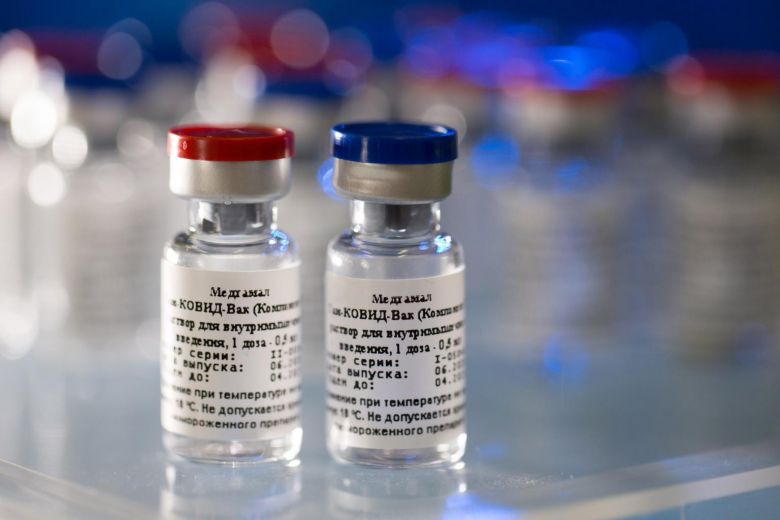
"Russia is essentially conducting a large population level experiment," said Ayfer Ali, a specialist in drug research at Britain's Warwick Business School.
She said such a super-fast approval could mean that potential adverse effects of a vaccine may not be picked up. These, while likely to be rare, could be serious, she warned.

Russian President Vladimir Putin said the vaccine, developed by Moscow's Gamaleya Institute, was safe and that it had been administered to one of his daughters.
"I know that it works quite effectively, forms strong immunity, and I repeat, it has passed all the needed checks," Putin said on state television.
"Mass vaccination with an improperly tested vaccine is unethical," he said. "Any problem with the Russian vaccination campaign would be disastrous both through its negative effects on health, but also because it would further set back the acceptance of vaccines in the population.”

In other news, the first cohort of local volunteers has been dosed with a coronavirus vaccine jointly developed by Duke-NUS Medical School and United States pharmaceutical company Arcturus Therapeutics.
Arcturus said in a statement on Tuesday (Aug 11) that this first phase of the trial involved volunteers aged between 21 and 55, who were injected with a single shot of the vaccine.
Data from this phase of the early-stage trial would be used to select the dosage regimens of the next phase, which will involve those aged 56 to 80, as well as younger adults.


